F2 Polar or Nonpolar Atom Closest to Negative Side
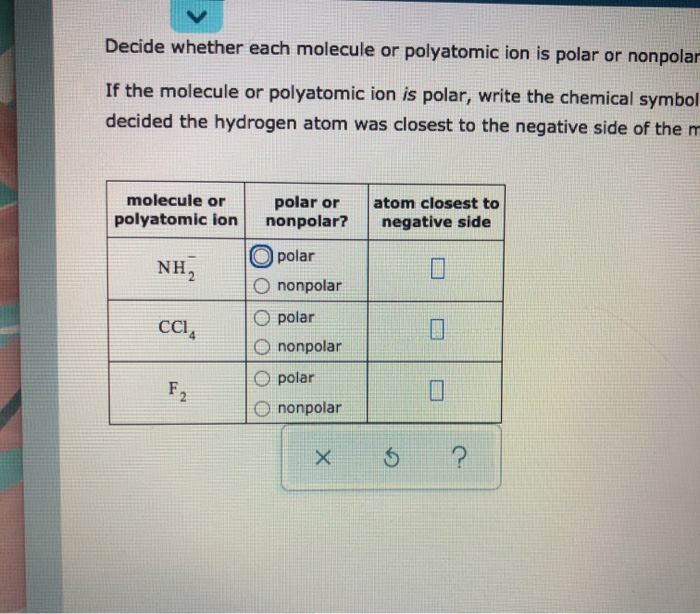
In chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Decide whether each molecule or polyatomic ion is polar or nonpolar.

Solved Decide Whether Each Molecule Or Polyatomic Ion Is Chegg Com
H2S is a slightly polar molecule because of its bent shaped geometrical structure and the small difference between the electronegativity of Hydrogen 22 and Sulfur 258 that results in a non zero dipole moment.
. It appears as a yellow colored gas. Since electrons carry a negative charge this atom will also have a partial negative charge on it. As the bond dipoles is not arranged symmetrically it is not a polar molecule.
If this were due entirely to the polar S-H bonds the S-H bond dipole would be at most 0. This net dipole moment in the molecule makes Chloromethane a polar molecule. Is NH3 polar or nonpolar atom closest to negative side.
Closest Atom Negative To Side F2 IBX9QO About Side Atom Closest Negative To F2 Daltons atomic theory - 5 postulates. Is not as high as elements like water ammonia etc. Electronegativity is the tendency of an.
Decide whether each molecule or polyatomic ion is polar or nonpolar. H2 and F2 is non-polar as they are made of two same atoms which me. The alkali metals IA the alkaline earth metals IIA halogens VII and the noble gases VIIIA.
For example if the molecule were HCl and you decided the hydrogen atom was closest to the negative side of the molecule youd enter H in the last column of the table. Boiling point -55C or 2201 F. HCl is a polar molecule and the chlorine atom closest to negative side because of electronegativity of the chlorine atom is higher than hydrogen so that it pulls shares pair of electrons from H atom as a result formation of partial positive charge on hydrogen and negative charge on chlorine atom.
Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. It easily reacts with metal ions to result in metal sulfides. Electronegativity plays a vital role in deciding whether the given molecule is polar or nonpolar.
Consider a hydrogen atom. In vacuum the singlet state spin zero atom has a lifetime of about 0. Ch4 has tetrahedral structure which is very symmetrical.
What is the atom closest to the negative side. What is F2 Atom Closest To Negative Side 0 m North side is the result of 2. 8 to get enough light from the flash at the basic output it is set to to achieve a correct exposure.
About Closest To F2 Negative Side Atom The overall molecule is Polar because the shape of the molecule is Trigonal Pyramidal which means it has the lone pair electrons. If the molecule or polyatomic ion is polar write the chemical symbol of the atom closest to the negative side. Four chemical families of the periodic table.
18b Ray 1 - Parallel to the principal axis after refraction by the lens passes through the focal point focus F2 of a converging lens or appears to come from the focal point F2 of a diverging lens. The proton side of the molecule in each case is positive. NH3 ammonia is indeed a polar molecule with an area of relative negatively charged at the top ie.
Molecule or polyatomic ion polar or nonpolar. For example if the molecule were HCl and you decided the hydrogen atom was closest to the negative side of the molecule youd enter H in the last column of the table. The filled free-electron pair orbitals are negative.
Because they are capable of hydrogen bonding. It is dangerous and toxic especially. Atom closest to negative side O polar nonpolar.
Answer 1 of 3. Atom closest to negative side polar nonpolar alba classify each molecule as polar or nonpolar. Other properties of H2S are.
This is because F is more electronegative than H and therefore F attracts the electrons of H to itself. If the molecule or polyatomic ion is polar write the chemical symbol of the atom closest to the negative side. The experimental structure of the co-crystallized ligands are also shown all atom colors carbons in magenta.
Decide whether each molecule or polyatomic ion is polar or nonpolar. Litotes isa two-component structure in which two negations are joined to give a positive evaluation. Laurence Lavelle completed his B.
What is polar and non-polar. 0 m West added together. Answer F2 Fluorine is Nonpolar.
Here is CH3Cl as Chlorine has more electronegativity than all the other atoms it has a partial negative charge and the hydrogen atoms have partial positive charges. Learn to determine if F2 is polar or nonpolar based on the Lewis Structure and the molecular geometry shapeWe start with the Lewis Structure and then use. I believe your misconception comes about from the asymmetrical shape part.
However it is higher than in many non-polar compounds. Normally in an atom the number of positively charged protons and negatively charged electrons is the same. If inhaled it can prove to be extremely fatal.
It is a toxic gas. F2 Side Closest To Negative Atom About F2 Side Atom Negative Closest To Such an atom having a net charge is called an ion. The lone pair electrons and a region of relative positive charge by the hydrogen atoms due to partial charges not being uniformly distributed throughout the molecule.
About Negative To F2 Side Atom Closest. About Atom F2 Closest Side Negative To. Normally in an atom the number of positively charged protons.
For example if the molecule were HCl and you decided the hydrogen atom was closest to the negative side of the molecule youd enter H in the last. This results in a polar bond. If the molecule or polyatomic ion is polar write the chemical symbol of the atom closest to the negative side.
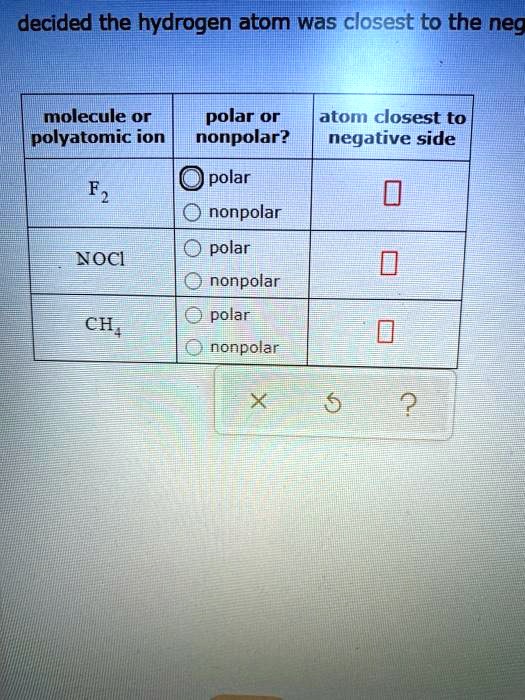
Solved Decided The Hydrogen Atom Was Closest To The Neg Molecule Or Polar Or Atom Closest To Polyatomic Ion Nonpolar Negative Side Polar F2 Nonpolar Polar Noci Nonpolar Polar Chj Nonpolar

Solved Decide Whether Each Molecule Or Polyatomic Ion Is Chegg Com

Is F2 Polar Or Non Polar Fluorine Gas Youtube

Solved Decide Whether Each Molecule Or Polyatomic Ion Is Chegg Com
No comments for "F2 Polar or Nonpolar Atom Closest to Negative Side"
Post a Comment